When you sell cosmetic products, the label is one of the most important things to get right.
It’s not just about making your product look good — it’s about complying with legal requirements and providing consumers with the information they need. While labeling requirmeents vary depending on where you sell your product, there are some best practices that are widely accepted.
By following these best practices, you’ll be ready to enter most markets without needing to make major changes to your label.
What is the MOQ for Private Label Skin Care Manufacturers?
Here’s a breakdown of the nine essential pieces of information that should appear on your cosmetic product label — regardless of where you intend to sell it.
Following these best practices will help you meet industry standards and ensure cosmetic label compliance in most regions.
1. Product Name and Function

Your cosmetic product’s name and function must be clearly stated on the label. This assures that consumers can immediately understand what the product is.
Even if the product seems straightforward, it’s crucial to include this information to avoid any confusion about the product or its purpose.
Additionally, the product name and function should always be presented in the language of the country where the product is being sold. This is a common requirement in many regions, ensuring that consumers can easily identify and understand the product in their native language.
2. List of Ingredients (INCI)

The ingredient list on a cosmetic product must include every ingredient used, listed in descending order by concentration. This means the ingredient that makes up the largest percentage of the product is listed first, and the smallest amounts are listed last.
The names of ingredients should follow the International Nomenclature Cosmetic Ingredient (INCI) system, which provides standardized names across different countries and markets.
For botanical ingredients, it’s often a good idea to include both the Latin name and the common name (e.g., Rosmarinus Officinalis for rosemary extract) to ensure clarity.
3. Name and Address of the Responsible Person (RP)

Your label must also include the name and address of the responsible person — the company or individual who brings the product to market. This is often not the manufacturer but rather the person or business that ensures the product meets legal requirements in the country where it’s sold.
For most readers, this means you are the responsible person.
Here are some key responsibilities of the responsible person for cosmetic products:
- Act as the main point of contact with authorities regarding the product.
- Handle any legal concerns related to cosmetic regulations in the relevant market.
- Address and resolve customer complaints related to the product.
- Monitor regulatory changes and ensure the product stays compliant with new laws.
- Manage any reports of negative effects or reactions from the product.
- Keep the Product Information File (PIF) updated and in line with regulations.
4. Net Weight or Volume
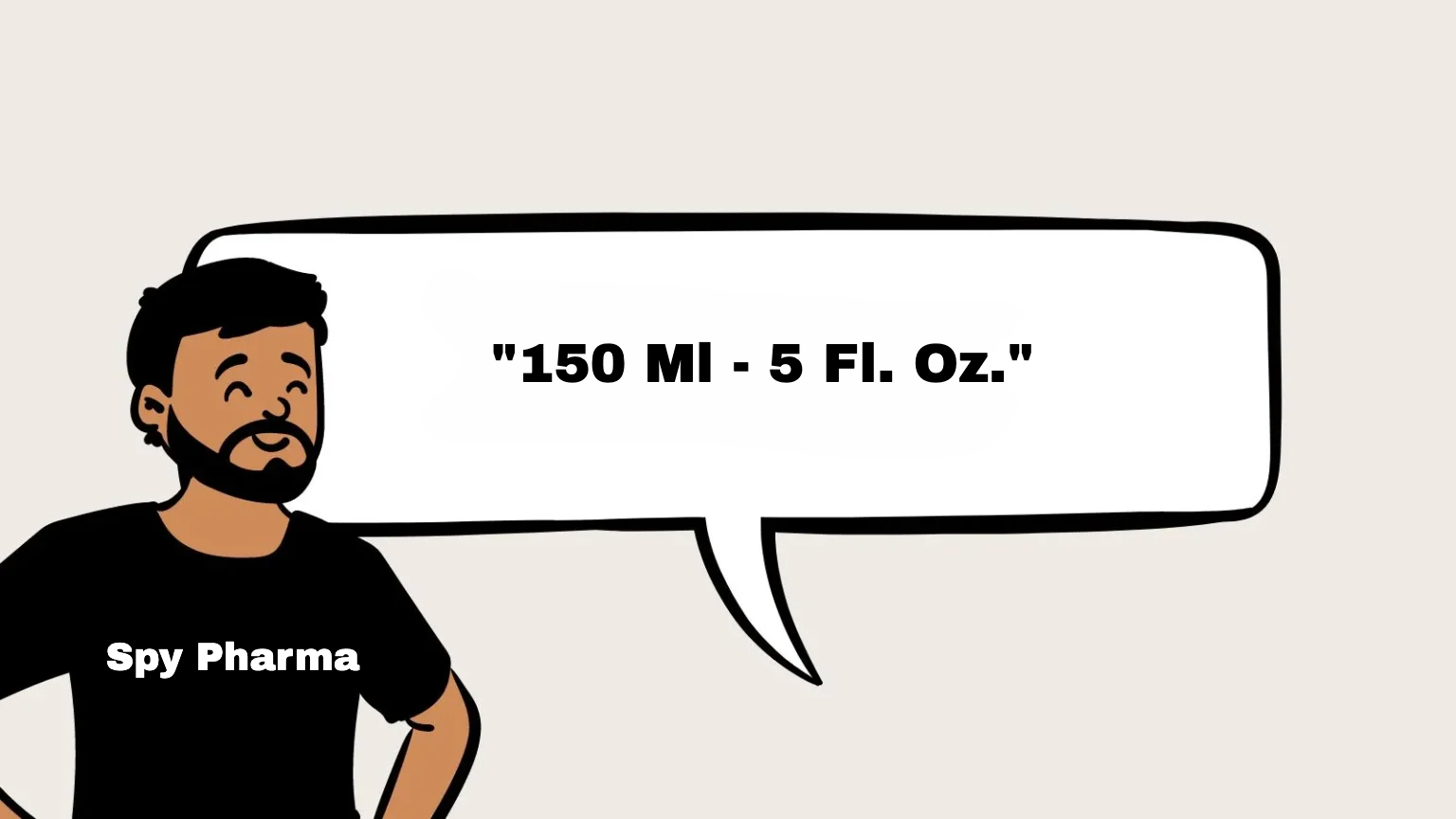
The net weight or volume of the product must be clearly displayed on the label. This tells consumers how much product they are purchasing, which is a key piece of information for any cosmetic item.
Including both metric and customary units (ml/fl oz, g/oz) on your label is the best practice. This ensures the product is easily compliant in both U.S. and Europe, as well as other international markets.
For liquids: Use both milliliters (ml) and fluid ounces (fl oz).
For solids or semi-solids: Include the weight in grams (g) and ounces (oz).
5. Date of Minimum Durability or Period After Opening (PAO)

For cosmetic products, it’s important to indicate either the expiration date or the Period After Opening (PAO) on the label, depending on the product’s shelf life.
Shelf life < 30 months: If the product has a shelf life of less than 30 months, you must display an expiration date. This is usually marked with an hourglass symbol or the words “Best before” followed by a date.
Shelf life > 30 months: For products with a shelf life of more than 30 months, the expiration date is not required. Instead, you must include a PAO symbol, which looks like an open jar with a number followed by “M” (for months). This shows how long the product is safe to use after it has been opened, such as “12M” for 12 months after opening.
Keep in mind that certain products, like single-use items or aerosols, may not need a PAO or expiration date, as their durability after opening is irrelevant. Make sure to check the specific regulations for your product type.
6. Batch or Lot Number
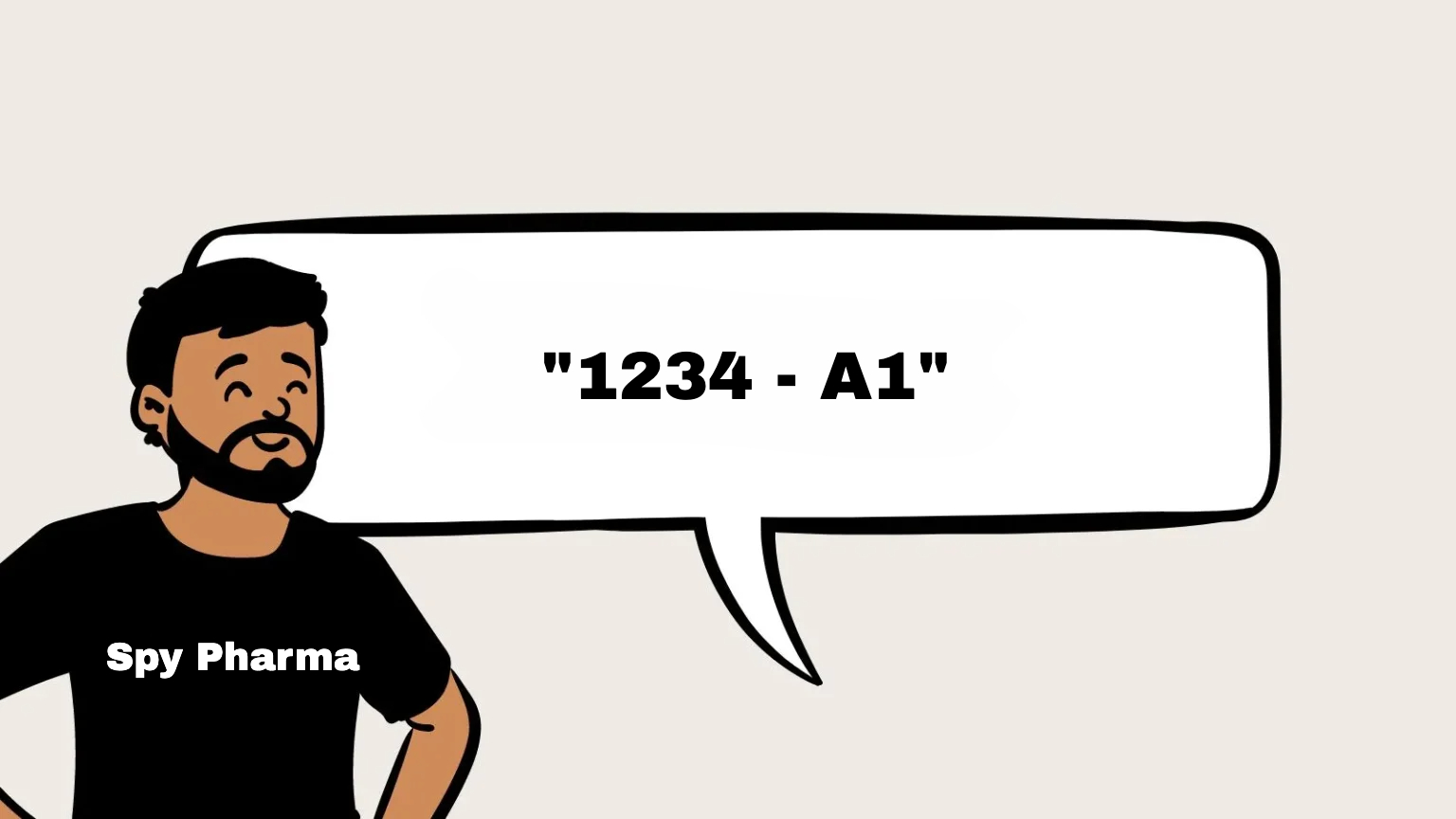
A batch number (also called a lot number) is essential for the traceability of cosmetic products.
It helps manufacturers track a specific batch of products, ensuring they can trace their manufacturing history, including the ingredients used, production date, and packaging process.
This is especially useful for quality control and recalls if any safety issues arise.
In most cases, you don’t have to worry about this. The cosmetic manufacturer of your products will simply print it on your products during production.
7. Directions for Use

Directions for use on your cosmetic product label is required by law in many regions, and they help your customers get the best results — especially for products where the proper application might not be obvious.
These instructions help consumers use the product safely and effectively. Directions should be clear, simple, and written in a way that anyone can easily follow.
If your product is more complex, like a chemical peel, it might need more detailed instructions than a regular shampoo, for example.
The FDA, for instance, requires this information to be displayed on a label in a conspicuous manner to ensure safety and prevent misuse
8. Warnings and Precautions

Some cosmetic products need to have warning labels because of certain ingredients or potential risks. The warnings should be easy to notice — usually printed in bold letters and placed on a contrasting background so they stand out.
For example, products with strong active ingredients like retinol, alpha hydroxy acids (AHAs), or beta hydroxy acids (BHAs), which are common in anti-aging and exfoliating products, can cause skin irritation, especially if overused or combined with sun exposure.
These products often include warnings like, “May cause skin irritation” or “Use sunscreen and limit sun exposure while using this product” to help consumers use them safely.
9. Country of Origin

When your cosmetic product is made outside the region where it’s sold, you need to include the country of origin on the label. This is required by law in places like the United States and the European Union and helps consumers know where the product comes from.
The country of origin is typically defined by where the product undergoes a “substantial transformation”. This means the place where the product changes its essential character or value the most.
Example 1
You are manufacturing a lip gloss.
The actual lip gloss (bulk) is produced in Italy, where it is also filled into the packaging. The packaging (the plastic bottle and applicator) comes from China.
In this scenario, you can definitely label your lip gloss “Made in Italy”, as the largest and probably most important part of the value creation takes place here.
Example 2
You are manufacturing a mascara.
The actual mascara (bulk) is produced in Italy. The packaging is imported from China, and the bulk is filled into the packaging in a third country, Germany.
In this case, determining the country of origin is not legally clear-cut and ultimately a matter of interpretation, whether it should be labeled as made in Italy or Germany.
For example, the Stuttgart Regional Court ruled that the mere assembly and quality control of PC components in Germany was not sufficient (Case No. 35 O 170/02) for the PC to be labeled as “Made in Germany.”
We haven’t found a similar ruling for cosmetic products. Filling the product in Germany could potentially be enough for a “Made in Germany” label if it is considered a sufficiently significant manufacturing step.
Our Top 5 Tips to Comply with Cosmetic Labeling Requirements
Now that you know the specific labeling requirements, please read these important tips.
1. Follow Best Practices, but Check Local Regulations
Most of the labeling practices mentioned in this post are widely accepted across the world, but cosmetic regulations vary depending on the country you’re selling in.
Following these best practices will help you enter many markets without making major changes to your label. Regions with strict labeling rules, like the European Union, set standards that many other countries follow or accept as well.
However, it’s always important to verify compliance locally.
To be sure that your labels meet all legal requirements, it’s a good idea to have a legal expert — especially someone familiar with the local market — review your labels. They can help you ensure compliance with specific regulations, verify translations, and make sure you’re meeting local language requirements.
2. Font Size & Contrast for Readability
Cosmetic labels must have a font size that is easy to read. For most products, we recommand the minimum font size is 1/16 inch (about 5 points (pt)), and for smaller packages, it can be 1/32 inch (about 2.5 points (pt)).
The text also needs to have good contrast with the background to be legible. With this color contrast checker you can check the contrast of two colors. You just have to insert the color of your text and background, and the tool is going to rate the contrast on a scale from 0–21. It also tells you if the contrast is good enough for small text.
If the text is too small or lacks contrast, it becomes unreadable, which is like not having the information at all. This can result in your product being considered non-compliant, since unreadable information doesn’t meet labeling regulations.
3. Keep Language in Mind When Expanding
One thing you should always keep in mind when expanding to new regions is the language requirement. Most countries will require that the information on the label be in the official language(s) of that region.
This means that while the content of your label may stay the same, you might need to add a translation when entering new markets.
4. Primary vs. Secondary Packaging
When deciding whether to include certain information on the primary packaging (the container directly holding the product) or the secondary packaging (like a paper box), the rules can vary depending on the region and the space available on the packaging.
As a rule of thumb, if there’s enough space on the primary packaging, all required information — like the product name, net weight, responsible person, PAO, batch number, direction for use and warnings — should be included there, even if it is also on the secondary packaging of your product.
5. Don’t Follow the Example of Big Brands Cutting Corners
While it might be tempting to follow what you see big brands doing, it’s important to know that many large companies don’t always comply with all labeling requirements — especially on their primary packaging.
Even though they often have enough space, some skip important information, likely for aesthetic reasons to keep the design clean and simple.
Smaller brands, on the other hand, don’t have that luxury.
In the cosmetics industry, smaller companies are often sued by competitors or face legal issues because their labeling doesn’t meet the necessary standards. This can lead to costly recalls or having to relabel your products — both of which can seriously hurt your business.
So, don’t cut corners just because the big brands do. It’s better to stay fully compliant with labeling laws and best practices to avoid legal trouble and protect your brand.
Get Your Labels Right: Save Time, Money, and Legal Trouble
Labeling your cosmetic product correctly is absolutely essential for staying compliant and avoiding costly legal issues. By ensuring your labels include the 9 key pieces of information, you’ll be prepared to sell in most markets without needing to constantly redesign your packaging.
Don’t take shortcuts.
Even if big brands sometimes skip certain details, smaller companies are at a greater risk of facing lawsuits or expensive recalls. It’s always better to be fully compliant from the start.
Need help with your cosmetic product labeling? Contact us for a free estimate. We are your one-stop solution for bringing cosmetic products to market. Whether you need private label services, custom formulation development, or packaging design, we’ve got you covered.
More Useful Guides
![White Label Cosmetics Explained: Pros & Cons in 2025 [+Guide]](https://www.spypharma.com/uploads/blog/white-label-cosmetics-explained.jpg)
White Label Cosmetics Explained: Pros & Cons in 2025 [+Guide]
![Top 17 Cosmetic Manufacturers for Beginners in 2025 [+Guide]](https://www.spypharma.com/uploads/blog/top-17-cosmetics-manufacturers-for-beginnerstop-17-cosmetics-manufacturers-for-beginners.jpg)
Top 17 Cosmetic Manufacturers for Beginners in 2025 [+Guide]

Low MOQ Cosmetic Manufacturers: Be Aware of These Dangers

Ultimate Guide for Vegan Private Label Cosmetics in 2024
![Top 9 Luxury Private Label Cosmetics Manufacturers [+Guide]](https://www.spypharma.com/uploads/blog/luxury-private-label-cosmetics-manufacturers.jpg)
Top 9 Luxury Private Label Cosmetics Manufacturers [+Guide]
![Starting a Lip Gloss Business: A Step-by-Step Guide [2025]](https://www.spypharma.com/uploads/blog/starting-a-lip-gloss-business.jpg)


